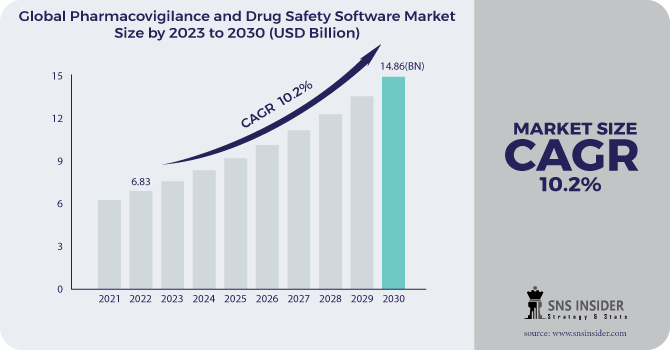
The global pharmacovigilance and drug safety software market size, valued at USD 6.83 billion in 2022, is projected to reach USD 14.86 billion by 2030, growing at a compound annual growth rate (CAGR) of 10.2% during the forecast period from 2023 to 2030. This significant growth is driven by the increasing focus on drug safety, stringent regulatory requirements, and the adoption of advanced software solutions to ensure compliance and efficient reporting.
Key Market Drivers
- Increasing Focus on Drug Safety: The rising incidence of adverse drug reactions (ADRs) and the need for efficient monitoring and reporting of these events are driving the demand for pharmacovigilance and drug safety software. Ensuring patient safety and minimizing risks associated with drug use are top priorities for healthcare providers and pharmaceutical companies.
- Stringent Regulatory Requirements: Governments and regulatory bodies worldwide are implementing stringent regulations for drug safety monitoring and reporting. Compliance with these regulations necessitates the use of advanced software solutions that can manage large volumes of data, streamline reporting processes, and ensure timely submissions.
- Adoption of Advanced Software Solutions: The adoption of advanced pharmacovigilance and drug safety software is increasing due to its ability to enhance data accuracy, improve workflow efficiency, and facilitate real-time monitoring. Features such as automated reporting, signal detection, and risk management are making these software solutions indispensable for pharmaceutical companies.
- Growing Pharmaceutical Industry: The expanding pharmaceutical industry, characterized by the development of new drugs and the increasing number of clinical trials, is fueling the demand for robust pharmacovigilance and drug safety systems. These systems are essential for ensuring the safety and efficacy of new drugs throughout their lifecycle.
Get a Free Sample Report of Pharmacovigilance and Drug Safety Software Market: https://www.snsinsider.com/sample-request/2379
Market Segmentation
The pharmacovigilance and drug safety software market is segmented based on functionality, delivery mode, end user, and region.
Functionality:
- Adverse Event Reporting
- Drug Safety Audits
- Signal Detection
- Risk Management
- Others
Delivery Mode:
- On-premise
- Cloud-based
End User:
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations (CROs)
- Others
Region:
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
Regional Insights
North America holds the largest share of the pharmacovigilance and drug safety software market, driven by the presence of major pharmaceutical companies, advanced healthcare infrastructure, and stringent regulatory frameworks. Europe follows closely, with strong regulatory requirements and a focus on patient safety. The Asia-Pacific region is expected to witness the highest growth rate, propelled by the growing pharmaceutical industry, increasing clinical trials, and rising awareness of drug safety. Latin America and Middle East & Africa are also showing promising growth potential due to improving healthcare infrastructure and increasing investments in the pharmaceutical sector.
Leading Companies
Key players in the pharmacovigilance and drug safety software market include:
- ArisGlobal
- Oracle Corporation
- Sparta Systems, Inc.
- AB-Cube
- Ennov Solutions Inc.
- United BioSource Corporation
- Veeva Systems Inc.
- Online Business Applications, Inc.
- Sarjen Systems Pvt. Ltd.
- EXTEDO GmbH
These companies are focusing on strategic collaborations, mergers and acquisitions, and continuous innovation to strengthen their market position and expand their product portfolios.
Future Prospects
The pharmacovigilance and drug safety software market is poised for substantial growth, driven by the increasing focus on drug safety, stringent regulatory requirements, and the adoption of advanced software solutions. Companies operating in this space are encouraged to invest in research and development to capitalize on the emerging opportunities and address the evolving needs of the pharmaceutical industry.
Other Reports You May Like:
Anxiety Disorder Treatment Market Size
Healthcare IT Outsourcing Market Size